However, it ought to be noted that the fact that a firm chooses to validate a course of action phase will not essentially outline that step as important.
Intermediate or API containers which have been transported outside of the producer's Regulate must be sealed within a method this kind of that, Should the seal is breached or missing, the recipient might be alerted to the chance which the contents may well are altered.
Collectively, APIs and drug products perform synergistically to deal with health care desires and improve affected person outcomes.
Validation of cleansing treatments should reflect actual machines use designs. If numerous APIs or intermediates are manufactured in the same devices as well as products is cleaned by exactly the same procedure, a agent intermediate or API might be chosen for cleaning validation.
is usually a raw substance, an intermediate, or an API that may be Utilized in the manufacture of an API and that is integrated as an important structural fragment into the framework from the API.
A key attributes of active pharmaceutical ingredients is their capacity to bind to receptors and elicit a physiological reaction that may also be advantageously Utilized in the cure of condition.
All specs, sampling ideas, and exam treatments really should be scientifically audio and appropriate to ensure that Uncooked materials, intermediates, APIs, and labels and packaging materials conform to set up standards of excellent and/or purity. Requirements and test treatments ought to be consistent with Those people included in the registration/filing.
Epinephrine: A hormone and neurotransmitter utilized being an emergency procedure for significant allergic reactions, bronchial asthma attacks, and cardiac arrest.
In just above a period of forty a long time, recombinant DNA engineering has developed to be one of several primary resources of new more info drug substances today.
There must be a published course of action that defines the instances underneath which a recall of the intermediate or API need to be viewed as.
The name of your producer, id, and amount of each cargo of each and every batch of Uncooked materials, intermediates, or labeling and packaging materials for API's; the name with the provider; the supplier's Management selection(s), if identified, or other identification selection; the quantity allocated on receipt; as well as date of receipt
Introducing unreacted substance back again into a approach and repeating a chemical reaction is regarded as reprocessing unless it is an element of your founded system.
In which the amount isn't fixed, the calculation for every batch size or rate of manufacturing must be integrated. Variations to quantities must be provided where by They are really justified
Validated analytical approaches having sensitivity to detect residues or contaminants needs to be applied. The detection Restrict for every analytical technique must be adequately delicate to detect the recognized appropriate volume of the residue or contaminant.
 Daniel Stern Then & Now!
Daniel Stern Then & Now!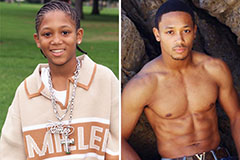 Romeo Miller Then & Now!
Romeo Miller Then & Now!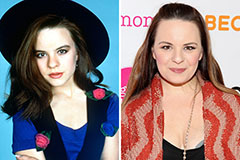 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Barbara Eden Then & Now!
Barbara Eden Then & Now! Dawn Wells Then & Now!
Dawn Wells Then & Now!